In gases the molecules are too widely separated or impacts are too
energetic, and in solids they are too constrained. Thus, the chemical
reactions essential to life probably have to take place in a liquid,
whether that is water or some other solvent.
Water is a polar solvent, that is, there is a slight difference in electrical
charge at the two ends of the molecule. Water is therefore able to dissolve
polar compounds, like sugar, salt, proteins, and nucleic acids. Our
chemistry reflects this in that proteins and DNA primarily undergo reactions,
whereas nonpolar lipids (fats and oils) act as insoluble membranes to
provide protected environments for reactions. If the solvent is nonpolar,
the situation would be reversed: lipids would float in solution and
would have to perform the same functions as our proteins do, and proteins
would be insoluble.
Life is so complex that it seems miraculous it could appear at all,
to say nothing of appear under extreme conditions. In recent decades,
life-as-we-DO-know-it has been found in far hotter, colder, or denser
environments than we thought possible. It has been found in rocks 600
meters underground, in Antarctic ice, and inside the boiling, radioactive
water of nuclear reactor vessels. It has become harder, not easier,
to imagine limits to the adaptability of life. Intelligence could develop
in any of these potential forms of life IF the reactions involved provide
enough energy, as discussed in the Metabolism
section below. Given the adaptability of familiar life, who is to say
what the limits of unfamiliar life might be? One of the easiest-to-read
introductions to the topic of physics, chemistry, and life is Asimov's
View from a Height. Although
dated at this point, the laws of chemistry have not changed and many
of his points are still valid.
Metabolism
Metabolism is the term for a series of chemical reactions that provide energy
in a form the organism can use for its own purposes: repairing cells, excreting
wastes, making new cells, and reproducing. In over-simplified terms, the energy
comes from an electron that is donated at the beginning of the process and
accepted by another atom at the end of the process. Anaerobic metabolism uses
a variety electron acceptors, but not oxygen. Aerobic metabolism uses oxygen
as the electron acceptor.
 |
Deinococcus
radiodurans,
an anaerobic bacterium capable of withstanding the radiation inside nuclear
reactor vessels. (Note that since this is a bacterium, the purple structures
are not nuclei, but some sort of cellular inclusions, as yet unidentified.)
Bacterial metabolisms have adapted to every environment containing any
water, including some almost as harsh as those expected on other planets
and moons in our solar system. (Electron micrograph: John Battista in "The
Planetary Report," Nov/Dec 2000. Scale: the larger purple inclusion
is approx 0.5 um across. At common screen resolutions the image is magnified
approx.50,000x.)
|
Anaerobic Metabolism
Although anaerobic metabolism seems exotic from our standpoint, it is
oxygen-based metabolism that is actually the strange and recent development.
Unlike the one method of aerobic metabolism, there are many different
anaerobic ones. Some of the electron-acceptors are sulfur, nitrogen,
methane, and other carbon compounds such as ethanol or lactic acid.
(Note: sulfur metabolism is not the same as hypothetical life based
on sulfur chemistry. In sulfur metabolism, the organism is still based
on carbon and water; it just uses sulfur somewhat the same way as we
use oxygen.)
Metabolisms whose end-products are ethanol and lactic acid are used by yeasts
and are also caled fermentation. Our cells use primarily aerobic metabolism,
but are capable of falling back on lactic acid metabolism when necessary.
When the body needs to rely on anaerobic metabolism to the point where lactic
acid builds up faster than it can be carried away, the acid builds up and
causes sore muscles. This happens when muscles receive too little oxygen for
the amount of work they have to do.
Bacteria have the widest diversity of metabolic systems, and among them,
the most diversity is found in the oldest bacteria, the Archaebacteria.
That implies there was considerable "experimentation" with
this most basic aspect of life in the anaerobic (oxygen-poor) environment
of early Earth. Some of these bacteria survive to this day in what are
now extreme environments where oxygen is unavailable, such as the extremely
saline muds of some salt marshes.
The most relevant point is that anaerobic metabolisms produce significantly
less energy than the aerobic kind. The energy-providing reactions are
not generally very tightly controlled in anaerobic metabolism. They take place
floating free in solution inside the cell. All anaerobic organisms are single-celled,
whether they are bacteria or more complex cells such as yeasts. Multicellularity
takes more energy than unicellularity because of the added energy needed to
transport materials around the organism, among other things. If anaerobic
metabolism does not provide enough extra energy to allow multicellular organisms
to develop, it is difficult to see how it is going to provide the even larger
quantities of energy needed for intelligence to develop.
Aerobic metabolism
To get an idea of how much more energy aerobic metabolism provides, consider
the outcome. The molecule that provides energy inside cells is ATP (adenosine
triphosphate), which breaks a bond to become ADP (adenosine diphosphate).
It then takes more energy from outside (i.e. from electrons cascading down
their chain, see diagram below) to rebuild it back to ATP. Anaerobic sugar
metabolism, as found in yeast, can make two ATPs from ADPs using the chemical
energy in one glucose molecule. Aerobic sugar metabolism, such as humans and
all other multicellular organisms on Earth have, produces 36 ATPs from
one glucose molecule. Imagine having to live, to say nothing of think, on
about one eighteenth of your usual energy. Even multicellularity costs too
much at that level.
The central element of aerobic metabolism, and the reason it provides
so much more energy, is a series of reactions in which an electron bounces
down a chain of molecules until it reaches a final electron acceptor
that is then excreted. It is similar to water falling onto a mill wheel,
except that chemical rather than gravitational energy is involved. In
aerobic metabolism, hydrogen is the electron donor at the beginning
of the process, and oxygen is the electron acceptor at the end. The
process is closely controlled by proteins embedded in membranes.
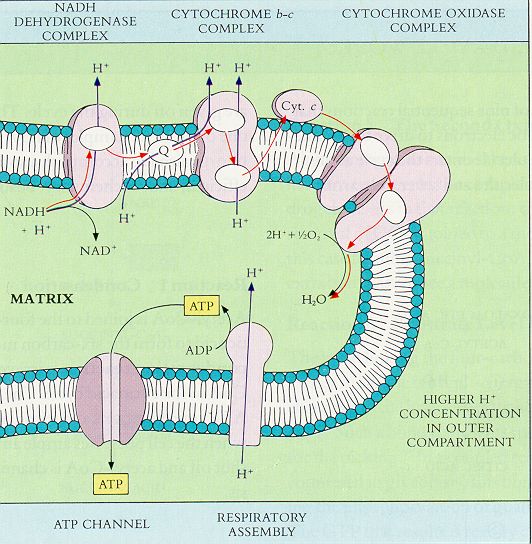 |
|
(from Raven &
Johnson, Biology, 1989, p. 171)
The
figure shows a slice through the membrane of a mitochondrion within
a cell. Mitochondria are organelles that produce energy (=generate ATP
from ADP) aerobically. The red arrows follow the cascading electron.
The electron starts at upper left when when NADH loses its "H,"
a hydrogen atom. The hydrogen's electron is passed through the first
carrier protein in the membrane (the oval blob). The protein pumps the
hydrogen's proton outside the membrane and passes the hydrogen's electron
to other carrier proteins within the membrane. These use the energy
of the "falling" electron to pump more protons out. Ultimately,
oxygen takes in 4 wandering electrons as well as 4 protons to make water.
(O2 + 4H+ + 4e- ->
2H2O)
Then comes the ATP energy-generating
part: All the protons (H+ ) that have been pumped
outside the membrane create a gradient, just like accumulating water
behind a dam. The protons "fall" back toward the inside of
the mitochondrion through special channels (keyhole-shaped membrane
structure at lower center). Each proton passing through the channel
provides enough energy to make one ATP out of a depleted ADP. Ultimately,
the ATP passes out of the mitochondrion through special channels big
enough to accommodate the big molecule and into the rest of the cell
where its energy is used and it turns back into ADP.
|
(Life in a nonpolar liquid, as discussed above,
might not be able to support electron transport chains. Life in a nonpolar
environment might therefore have the same energy limitations as anaerobic
life does in our environment.)
Biochemical Variations
Variations in basic chemistry and metabolism are based on the relatively
small molecules of life. But the big macromolecules, the DNA, proteins,
carbohydrates, phospholipids, glycosides, and so on, fundamentally structure
the biology of the organisms dependent on them. We have only one model
of biochemistry, the one evident on Earth, which makes it difficult
to understand the specific influence of any one aspect of biochemistry.
Science fiction, which has helped expand thinking on what forms life
might take under alternate gravities and chemistries, is much less prolific
on this subject. The tendency is to assume that substituting one type
of protein for another would be much like using concrete blocks instead
of bricks to make a building. The material is different, but the resulting
structure is functionally the same. This assumption may be justified.
More likely, as we understand the structural and other implications
of different macromolecular conformations, it will turn out that different
macromolecules result in different cell structures and physiologies.
That, in turn, would result in very differently structured organisms.
This topic has received less attention than some of the other ones in
this unit, so the Assignment focuses on a macromolecular question.
Questions/ Assignments
Oxygen is toxic to all organisms, including the aerobic ones that depend
on it, because of its extreme chemical reactivity.
Assignment: Joan Slonczewski's The
Children Star depicts a world that is strange from a molecular
standpoint. The dominant life forms are prokaryotic, that is, like the
bacteria on our world. What adaptations do the prokaryotes have to either
increase the energy yield of their metabolism or to compensate for low
energy?
The genetic material is DNA, but it is in triple helices, not double-stranded
like ours. How would this affect DNA replication? What would the implications
be for the speed of cell replication and hence growth on this world?
The amino acids that make up the proteins are different. On Earth, amino
acids that are not normally found in proteins may be toxic, indigestible,
or harmless. Prokaryon's biomolecules are toxic to humans, but humans
can be genetically engineered to tolerate them. What is the likeliest
form of this genetic engineering? Enabling humans to use the amino acids,
i.e. altering our biochemistry? Making human membranes impervious to
them? Engineering enzymes capable of neutralizing them? Some other solution?
What are the pros and cons of different solutions?